Abstract
Background: AML is an aggressive clonal hematopoetic disorder that requires early initiation of therapy. Intensive induction therapy with cytarabine plus an anthracycline (ie, 7+3) is the mainstay of initial treatment for fit patients with AML; however, in elderly patients there are no validated criteria with which to define fitness (Döhner. Blood . 2017). While prognostic indices may predict therapy response, no consensus exists regarding optimal therapy in these patients. We sought to explore characteristics and survival outcomes of elderly AML patients (age >=60 years) treated with 7+3 vs less intensive therapy with HMAs (ie, azacitidine and decitabine) in a real world setting.
Methods: Elderly(>=60 years of age)AML patients that initiated frontline therapy with either 7+3 (cytarabine with either daunorubicin or idarubicin) or an HMA (azacitidine or decitabine) were retrospectively identified from a large US electronic medical record from 1/1/2008-7/31/2015. AML diagnosis included >=1 inpatient or >=2 outpatient records with an AML ICD-9/10 code (the first record was the index date). First-line therapy (1LT) was defined as an AML-specific treatment initiated on/after the index date; a switch in agent triggered second-line therapy (2LT). Patients were followed until death, end of follow-up, or end of study (9/31/2015). The number of unique 3-digit diagnoses and those used to calculate the Charlson Comorbidity Index (CCI) score within the baseline period were captured. At 2 years following initiation of 1LT, median overall survival (OS) and progression-free-survival ([PFS], defined as time from initiation of 1LT to 2LT or death) and OS and PFS rates were evaluated using Kaplan-Meier analyses.
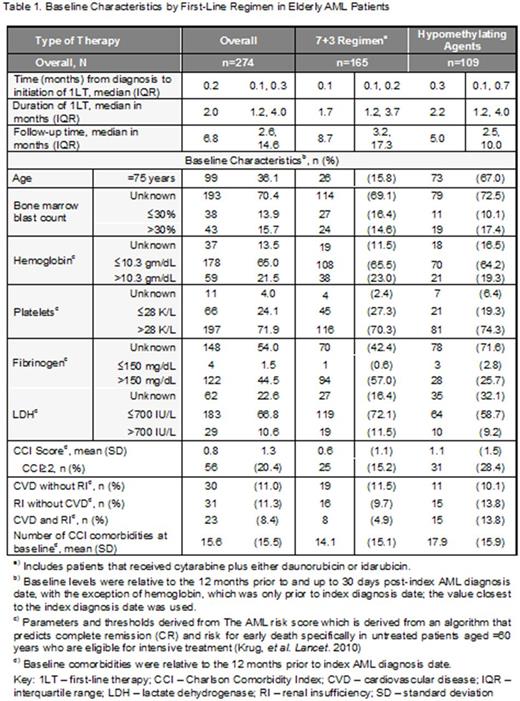
Results: Of 704 eligible AML patients, 274 received 1LT with either 7+3 or an HMA. Mean age was 71.5 years (SD: 7.1), 55.8% were male, and 20.4% had a CCI score of >=2. A higher proportion of patients that received an HMA were ³75 years of age compared to those that received 7+3 (67.0% vs 15.8%, respectively), and a lower proportion of patients that received HMAs had platelets <=28K (19.3% vs 27.3%, respectively). Patients receiving HMAs also had higher mean number of unique comorbid conditions at baseline (14.1 [SD: 15.1] vs 17.9 [15.9]) and a higher proportion of those on HMAs had comorbid cardiovascular disease (CVD) with renal insufficiency (RI) (13.8% vs 4.9%). Patients on a 7+3 regimen had a longer median PFS and higher 1- and 2-year PFS rates than those on HMAs: median: 6.7 months (95% confidence interval [CI]: 4.9, 11.1) vs 4.1 (95% CI: 2.8, 4.9); 1-year PFS: 41.6% vs 21.3%, P <0.01; and 2-year PFS: 30.4% vs 9.9%; P <0.01). Median OS was also longer and 1- and 2-year OS rates were higher in the 7+3 cohort vs the HMAs; median OS: 14.7 months (95% CI: 11.0, not reached) vs 4.3 months (95% CI: 3.2, 5.8); 1-year OS: 55.9% vs 23.8%, P <0.01; 2-year OS: 42.8% vs 11.3%, P <0.01. Despite available literature suggesting HMAs are better than supportive care in terms of PFS, majority of the patients do not appear to be receiving any therapy.
Conclusions: Elderly AML patients treated with 7+3 had better outcomes in terms of PFS and OS than those treated with HMAs in our retrospective analysis. Age >=75 years, number of comorbid conditions, and presence of CVD with RI in this population appear to be associated with a propensity to administer HMAs over the more intensive 7+3 regimen. The outcomes with HMAs are lower than that suggested by available data from clinical trials likely due to differences in patients in clinical trials vs. the real-world setting. More research is needed to evaluate other factors in therapy selection and improve outcomes for the elderly AML population.
Bell: Takeda Pharmaceuticals: Employment, Equity Ownership. Galaznik: Takeda Pharmaceuticals: Employment, Equity Ownership. Farrelly: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Xcenda: Employment. Murty: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Xcenda: Employment. Ogbonnaya: Xcenda: Employment; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy. Eaddy: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Xcenda: Employment. Fram: BeyondSpring Pharmaceuticals, Inc.: Consultancy; Takeda Pharmaceuticals: Consultancy. Faller: Takeda Pharmaceuticals: Employment, Equity Ownership. Kota: Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; Xcenda: Consultancy; Leukemia Lymphoma Society: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal